
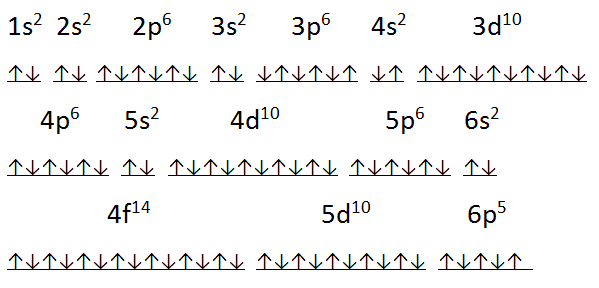
In simple terms, it means that every time, electrons are first filled up in an orbital singly and only when needed, do the electrons with opposite spin pair up.
“ For a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity”. This also limits the number of electrons occupying an orbital to two. It essentially means that if electrons must occupy an atomic orbital, they must have opposite spin. “ No two electrons have the same 4 quantum numbers n, l, m, s”. Read more about Electron Structure The Pauli Exclusion Principle: There are 3 rules which need to be followed for the accurate prediction of the electronic configuration.

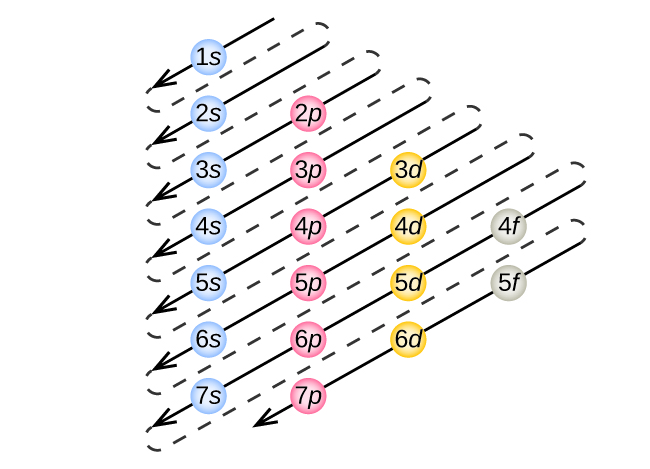
Filling up of the electrons and the resulting electronic configuration of an element is governed by some rules which are pivotal to the understanding of the chemical processes. The arrangement of electrons within an atom is called the electronic configuration and the electrons are filled up according to the energy of the levels as: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f. The order of increasing energy of orbitals as shown below, is backed by experimental data. The electrons in the 3d orbitals are not able do this and hence are higher in energy. It is expected that the 3p subshell will be followed by 3d, but it is really the 4s which follows. Although their energies are close, 4s is lower in energy because of electrons in this orbital being able to move close to the nucleus due to the symmetrical spherical shape of the s orbital. Its observed that everything looks correct until the 3p subshell is passed. The inter-electronic repulsion inside the atoms is the cause of the chaotic order of the orbital energies. As a replacement, we would consider a diagrammatic representation along with the physical view of the arrangement of electrons. Herein starts the anomalous behaviour which is a result of the complex mathematics which are avoided here.
Principal quantum number n determines the energy of the orbitals while angular momentum (azimuthal) quantum number determines some of its energy and largely its shape. Orbitals are the region around the nucleus in which there is maximum probability of locating an electron. For example, a 3s orbital is lower in energy than a 3p orbital which is lower in energy than a 3d orbital. It determines the shape of the orbitals and the energy of each level within a given principal quantum number. The other identifying feature of an orbitals for their representation as s, p, d, and f, is the angular momentum quantum number (l). Similarly, as the value of n increases, the energy of the electrons residing in those levels also increases. The electrons in an energy level bearing n = 1 have less energy than those in level having n = 2 or more. The numbers represent the principal quantum number (n) or the “energy levels”. For usage in various applications, these orbitals are labelled as s, p, d, and f with precursors 1, 2, 3, 4, 5, 6, 7 ……and so on. Since the orbitals are only telling us about the probability of finding an electron, it’s imperative to be as sure as possible about the whereabouts of the electron inside an atom. The Heisenberg Uncertainty Principle says – “ it is impossible to define with absolute precision, at the same time, both the position and the momentum of an electron” which rules out any definite orbit. To be able to assign a definite path, it’s absolutely necessary to know the location of the electron at an instant and to be able to predict its movement the next instant. Although from a simplistic view, electrons may look like going around the nucleus in a similar way, in practice, they populate regions of space which are called orbitals. Orbitals and orbits – the differenceĪ planet moving around the sun in a fixed path is said to be moving in an orbit. In order to understand the nature of electronic configuration, we need to understand the terms orbitals and energy levels. The very first criteria to explain the chemistry of any species, is to identify the arrangement of electrons because, the chemical reactions are essentially the rearrangement of electrons. Further development of chemistry, especially in the sphere of quantum mechanics, has enabled us to rationalise the existence of all elements in modern Periodic Table and their arrangements are applied to elucidate the chemical properties. In this endeavour, Mendeleev put forward the periodic table of elements. Since the very early days, chemists have tried to organise the elements so that their properties can be studied with minimum of effort. Which quantum number defines the energy of orbitals?. What do you understand by the term “Orbital?”.







 0 kommentar(er)
0 kommentar(er)
